GAC Sorption Process:
Problems and Solutions
By K. Vasanth Kumar, K. Subanandam, V. Ramamurthi and S. Sivanesan
March 2004
The Authors are Research Scholars at the Department of Chemical Engineering - A.C. College of Technology, Anna University, in Chennai - India. → See also:
Granular Activated Carbon (GAC) process is suffered by many disadvantages like competitive adsorption and fouling and also on the cost of adsorption process. These problems also interferes with mass transfer/kinetic behavior of solute in sorbent causing a major problem in adsorber design. In the present discussion, the various problems that arise during adsorber design were discussed and some solutions were provided to tackle these problems.
Key words: adsorption, GAC, competitive adsorption, fouling, cost
Introduction
Currently GAC sorption process is proved to be an attractive and effective process in wastewater treatment for removing color, heavy metals and organic compounds. However this process is expensive due to high cost of GAC and regeneration cost. Apart from these, there are also some other problems such as competition and fouling that arise during the operation.
The main objective of our present communication is to give an outline of adsorption theory and mechanism associated with sorption process and to identify the problems associated with its kinetics and mechanistic steps. Also a simple solution chart is prepared to compromise these problems associated with the sorption systems.
Defenition/Theoretical
Sorption process is defined as the selective transfer of solute from the bulk solution onto the surface of the adsorbent. The solute transfer process is assumed to proceed through the following three mechanisms:
- Movement of solute from the bulk solution onto the surface of adsorbent.
- Migration of solute from the relatively small area of external surface to the pores within the pores of each adsorbent particle. Majority of adsorption occurs in the pores, because of the more availability of surface area (m²/g).
- Solute molecules adhere to the surface in the pore and finally solute transfer process terminates after equilibrium.
Major Problem
Various models were available to determine the rate constant corresponding to these three different steps. However not even a single relationship is available combining the three mechanisms involved or to predict which is the rate controlling step, causing a major problem in adsorber design.
However based on different ideas available in literature, it is possible to determine the nature of sorption process. For example, if the pore diffusion coefficient (cm²/s) values fall between 10-11 to 10-13, then the process is said to controlled due to intraparticle diffusion coefficient ¹. Similarly if the external diffusion coefficient value falls in between 10-5 to 10-8, then the process is said to follow external mass transfer 2,3. For temperature controlled systems, if the activation energy falls less than 42 KJ/mol then the process is confirmed to be diffusion controlled, and if it falls above 42 KJ/mol the sorption process is due to chemisorption.
Problems With These Models and Equilibrium Approach
As said before, due to varied mechanism involved in sorption process, mass transfer rate in pores and surfaces are difficult to treat. Also different models were available to predict the sorption process, it is also a time consuming process to check the applicability of model for any single solute sorbate systems. Also a lot of time consuming experimental investigations and data were required to predict the nature of the sorption process, unless it is possible to predict the nature of sorption process, unless it is possible to predict the nature of sorption process 2,4,5.
Also most of these models will work based on the assumption of adsorbents as true homogeneous spheres. But not even a single adsorbent particle is proved to be true homogeneous at microscopic level.
Equilibrium approach: Due to these difficulties associated in predicting the nature of sorption process, empirical design procedures based on adsorption equilibrium conditions have been commonly used to predict the adsorber size and performance. The different parameters and the underlying thermodynamic assumption of these equilibrium models often provide some insight into both the sorption mechanism and the surface properties and affinity of sorbent. The most commonly used equilibrium models are Freundlich, Langmuir, Redlich Peterson, Dubinin Raduskevich, Temkin and Sips isotherm models etc. Equilibrium data well representing the Freundlich isotherm represents the expectation of multilayer sorption. Also it confirms the sorption process violating Henrys law at lower initial solute concentrations. Similarly the equilibrium representing the Langmuir confirms the monolayer coverage of solute particles onto sorbent particles and also the equilibrium curve follows Henrys law at lower initial solute concentrations. Apart from Freundlich and Langmuir isotherm model, the most often-used equilibrium model is Redlich Peterson model, which incorporates both the advantageous significance of both the Freundlich and Langmuir model. Redlich Peterson isotherm equation represents the equilibrium curve to follow Henrys law and the curve behavior follows Freundlich isotherm equation at higher initial solute concentrations. Another equilibrium model derived from the limiting behavior of Freundlich and Langmuir equilibrium model is Sips or Langmuir-Freundlich model. This model suggests equilibrium data follow Freundlich curve at lower initial solute concentration (i.e. it violates Henrys law) and follow Langmuir pattern at higher solute concentrations. The another most commonly used equilibrium model to predict the sorption nature is Dubinin Raduskevich model and it is used to calculate the mean free energy of sorption as it is transferred to the surface of solid from infinite distance in the solution. Also the results were mainly used to identify whether chemisorption is involved. If the calculated bonding energy falling between 8 - 16 KJ/mol, indicates the chemisorption may play a significant role in the adsorption process. The dependence of temperature on equilibrium capacity can be identified based on the heat of adsorption value using Temkin isotherm equation. The best fit of equilibrium data in Temkin isotherm equation indicates the heat of adsorption is liner rather than logarithmic.
Though a number of models were available, it is the duty of designer to predict/select the best equilibrium model. A best fit model can be predicted using linear regression correlation coefficient values or some other error estimation techniques 6.
Advantages: The main advantages of equilibrium approach includes:
- It reduces the work (monitoring) load.
- Easier interpretation of experimental data, as it doesn´t considers the complex mechanistic part involved before the attainment of equilibrium stage.
- Easy to interpret the nature of adsorption process, as each equilibrium model rests on a definite mechanical/theoretical assumption.
Practical Problems Associated With Adsorption
In practice, the three-step mechanism gets disturbed due to the following two factors:
- Competitive adsorption: Competitive interaction between different adsorbates (COD, color, heavy metals, organic compound etc) with adsorbent. The interaction of different adsorbates will reduce the sorption capacity of the adsorbent. The interaction of different adsorbates will reduce the sorption capacity for the particular pollutant to be removed or the target compound. Competitive inhibition also interferes with mass transfer kinetics of adsorbent and adsorbate. Also there doesn't exist any kinetic, dynamic and equilibrium model to better explain competitive inhibition.
- Fouling: Presence of any sediments or suspended matters, which in turn will show an impact in diffusion rate, resists the intraparticle penetration due to clogging of pores. Also these will have direct impact on mass transfer and kinetics of sorption process.
Thus estimating the significance of competitive inhibition and fouling factors for a particular field condition, it is possible to evaluate its impact on the adsorption capacity and kinetics of solute compound to be removed. However this will once again will create extra workload to generate experimental data. Also all these kinetic and equilibrium models will fail to explain the sorption process, if fouling due to suspended particle interferes with the process.
Solution to Multicomponent Adsorption
If the GAC adsorption process is influenced by competitive inhibition the following considerations will improve the sorption process:
Optimize system for target compound: In case of multi component sorption or the influence of foreign compounds on target compound, then go for optimization of design variables for the target compound. Target compounds should be selected based on the following considerations.
The weakly adsorbing adsorbates that are liable to breakthrough from the adsorber at the earliest should be selecterd as the target compound. This early breakthrough potential will depends upon the pollutant concentration as well as their adsorption capacity. This will avoid the competition of other foreign compound, which has higher breakthrough time at later time.
Target compounds should be chosen, only if they are to be regulated. Supposing an organic chemical that is not regulated is identified as the chemical that will breakthrough early from the adsorber, still it should be chosen as target chemical.
Adsorbent size: If competitive adsorption is expected, then decreasing the particle size will increase mass transfer rate of sorbate. However very fine particles cannot be used in fixed beds. McKay 4 found that mass transfer coefficient values were found to vary with dp0.046 for telon blue onto wood as:
K = 1.5 x 10-4 dp0.046 [1]
Agitation: For well agitated systems, it is proved that agitation may enhance the mass transfer kinetics. Higher agitation speed will also decrease the clogging of adsorbent particles by suspended solids. McKay 4 found that K values tend to vary with RPM0.046 for telon blue onto wood as:
K = 1.624 x 10-4 dp0.18 [2]
Type of GAC: Different type of adsorbents derived from different source can be used to adsorb specific adsorbate or target compound. The best adsorbent for a specific target compound can be selected based on higher adsorbent capacity of the adsorbents for the target solute to be removed. However previous (laboratory) investigations were required to explore the adsorption capacity of adsorbents for the target compound.
EBCT: For the target compound with faster breakthrough time, adsorber with larger empty bed contact time (EBCT) yield larger specific through put or in other words the volume of water treated per unit mass of GAC increases. This will reduce the competition of foreign compounds. However the competeting adsorbates should obtain breakthrough time, tb greater than that of target chemical.
pH: Optimize the solution for pH for target compound. This is the simplest way to minimize the competition of foreign compounds over the target compound.
Some Solutions To Fouling
Type of adsorbent: Adsorbents other than GAC can be used. Macro-Reticular resins and molecular sieves have been shown to be resistant to organic matter fouling may in long prove to economical, though they are more expensive than GAC.
EBCT: Though adsorber will yield increasing specific through put with EBCT when the adsorption process is controlled by competition, the specific throughput will start to decrease after the adsorbent exceeds a critical EBCT due to fouling. For practical cases, an adsorber with an EBCT to 9 to 15 minutes will yield largest throughput. (For most of organic materials, target compounds removal % should fall within these EBCT).
Pretreatment: Implementation of sedimentation process as pretreatment step to remove suspended solids from wastewater before subjecting to sorption process will resist the clogging due to fouling.
Making GAC Economic
Apart from multicomponent sorption and fouling, the major problem with the sorption process is the cost of GAC and cost involved in regeneration and operation.
Decide regeneration/disposal: In certain applications it may be more economical to discard the adsorbent after use. Disposal would be favored when the adsorbent is of low cost, is very difficult to regenerate, and the non-adsorbed component is the desired product of very high value. In the majority of applications, the disposal of adsorbents as waste is not an economic option and therefore regeneration is carried out either in situ or external to the adsorption vessel to an extent that the adsorbents can be reused.
Adsorbent regeneration: Practical methods of desorption and regeneration include one, or more usually a combination, of the following: Increase in temperature, Reduction in partial pressure, Reduction in concentration, Purging with an inert fluid, Displacement with a more strongly adsorbing species, Change of chemical conditions. The final choice of regeneration method(s) depends on technical and economic considerations.
Cyclic adsorption process (pH Swing Cycle): When the discharge of effluents is of large quantity, continuous cyclic sorption process is always effective and cost affordable. All adsorption processes use changes in temperature, pressure, concentration of a competitively adsorbing component to effect adsorption and desorption. But presumably any other variables, which could effect changes in the shape of an adsorption isotherm, could also be used. One such variable is the pH. The bonding between some adsorbents and adsorbates such as amino acids in water can be changed remarkably as the pH is changed from above the isoelectric point of the amino acid to below its isoelectric point. The isoelectric point is the pH at which the amino acid molecule has zero charge. The economic problem of using pH swing as a means to drive a cyclic process is the cost of the acid and base required to change the pH, as well as the cost of disposal of the salt by-product.
For highly conductive adsorbent and adsorbate: Another means for changing the shape of the adsorption isotherm is the use of electric charge. Electrosorption involves adsorption when the adsorbent is subjected to one voltage and the desorption when the voltage is changed. Typically the voltage can be small, such as 1V or less. This process can only be accomplished in cases in which both the adsorbent and the feed stream are highly conductive. E.g.: EDA (ethylenediamine), which demonstrates different loading at different voltages.
Carbon refilling: Though liquid drainage is clearly downwards, it is preferable to refill an adsorbent bed in the upward direction because it is easier to sweep out pockets or gas or vapor and so prevent misdistribution in the proceeding adsorption step. Consideration must be given to the time required to ensure that the gas and pockets have been removed completely otherwise there is a risk that they will contaminate the product in the adsorption step and cause excessive lifting of the bed.
Type of contact: Adsorption process become affordable based on the type of contact system choosed. Normally five type of contact system can be made. They are fixed bed, pulsed bed, moving bed, fluidized bed and batch operation. Normally batch operations are selected only if the volume of wastewater to be treated is very low. Similarly continuous operations are selected if the there is a continuous discharge of effluents of large volume per day by the industries to the receiving streams.
Conclusions
Considering all these problems, a simple solution chart is prepared which may help in economic and effective design of adsorbers. Let the wastewater shown in Figure 1 may contain both target compounds and foreign compounds. The first step is to identify the target compound, which is selected based on solute having lower breakthrough time in case of continuous column or solute having earlier breakthrough time in case of batch experiments. After predicting the target compound, the next step is to identify the best sorbent which is selected based on the sorbent having maximum sorption capacity of sorbent. After identifying the suitable sorbent, then optimize the solution pH for the target solute and adsorbent. Then go for the sorption process. Based on the experimental values, predict the mechanistic aspects involved in sorption process using various kinetic/transport models. If the kinetic approach collapses the decision then go for the equilibrium approach. Then finally predict the design equation. However equilibrium approach is highly satisfactory it will give only the details on amount adsorbed onto adsorbent. Its only the kinetic data that will be useful in predicting the volume of reactor. Though its possible to predict roughly the adsorber size based on the adsorption capacity and the volume of water to be treated per unit time, it is not proved in literature for industrial appalications.
Figure 1: Solution Chart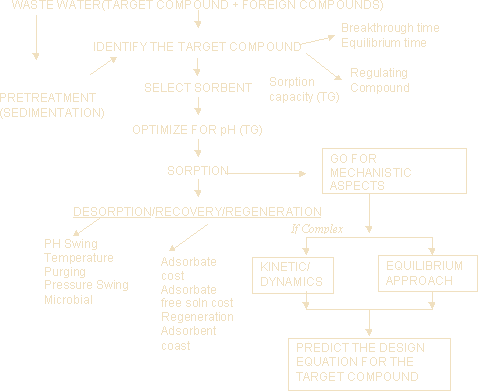
After completion of sorption process, decide for regeneration/disposal of adsorbents, which is to be considered based on the cost of solute, cost of sorbate free solution and also on the regeneration cost.
References
- B.S. Inbaraj and N. Sulochana, Basic dye adsorption on a low cost carbonaceous sorbent, Indian journal of chemical technology., 9, 201 (2002).
- T. Furusuwa and J.M. Smith, Intraparticle mass transport in slurries by dynamic adsorption studies. AiChE., 20, 88 (1974).
- S.K. Khare, K.K. Pandey, R.M. Srivasatava and V.N. Singh, Removal of Victoria blue from aquepus solution by fly ash, J. Chem. Technol. Biotechnol., 35, 99 (1987).
- G. McKay and I.F. McConvey, The external mass transfer of basic and acidic dyes on wood, J. Chem. Technol. Biotechnol., 31, 401 (1981).
- S. Ismadji and S. K. Bhatia, Adsorption of Flavour Esters on Granular Activated Carbon, Can.J.Chem.Engg., 78, 892 (2001).
- Y.S. Ho, J.F. Porter and G. McKay, Equillibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, Nickel and Lead single component systems, Water Air and Soil Pollution., 141, 1 (2002).
- J.D. Seader and E.J. Henley, Separation Process Principles, John Wiley Publications, Singapore (1999).
- W.J. Thomson, Introduction to Transport Phenomena, Pearson education Asia, India (2001).
***
Copyright © 2004, ECO Services International